2019 Drug Review Annual Report Is Released
On July 30, 2020, the Center for Drug Evaluation released the 2019 Drug Review Annual Report, some contents of which are summarized as follows:
As a milestone in the history of China's legal system development in drug administration, the year of 2019 saw the newly formulatedVaccine Administration Law, marking the first comprehensive law for vaccine administration in the world, and the newly revised Drug Administration Law, marking an across-the-board revision in the past 20 years, all of which symbolizes, in the form of the law, the crystallization of the deployment of the Party Central Committee and the State Council, the expectations of the people and the experience of review system reform, providing a powerful legal safeguard to consolidate and promote the reform of the drug review and approval system. In 2019, the Center for Drug Evaluation (hereinafter referred to as CDE), under the strong leadership of National Medical Products Administration (hereinafter referred to as NMPA), carefully studied and implemented the Drug Administration Law and theVaccine Administration Law, continued to promote the reform of the drug review and approval system, actively promote scientific management system through process streamlining for drug review, adhered to law-based, open&transparent, servicecentered and scientifically standardized review, to effectively guarantee the safety, effectiveness and accessibility of drugs, and protect the people's health rights and interests.
Acceptance of drug registration applications
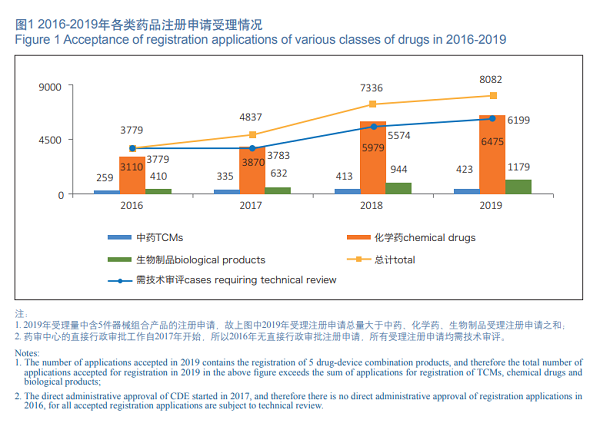
In 2019, CDE accepted a total of 8,082 new registration applications (including 5 drug-device combination products, counted by acceptance numbers, the same below), of which 6,199 applications are subject to technical review (including 4,907 applications subject to technical review and administrative approval of CDE), and the rest of 1,878 applications are subject to direct administrative approval (not requiring technical review, the same below).
Overview
Of the 8,077 drug registration applications accepted by CDE, 80.2% (6,475) of the total are chemical drugs applications in 2019. See Figure 1 for the acceptance of registration applications for various classes of drugs in 2016-2019.
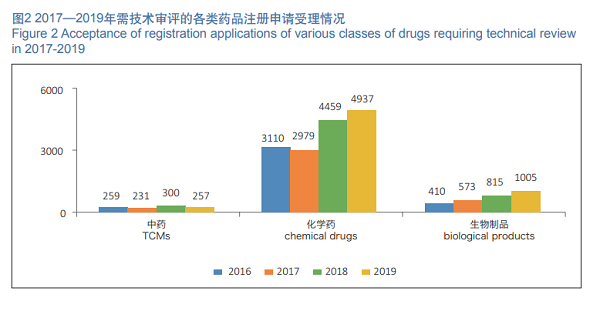
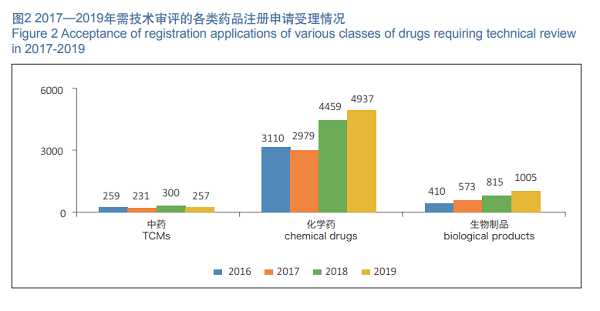
In 2019, 6,199 registration applications requiring technical review were accepted, increased by 11.21% as compared with those in 2018. Among them, 4,937 registration applications were for chemical drugs, increased by 10.72% as compared with those in 2018, accounting for 79.64% of the total number of registration applications requiring technical review; 257 registration applications were for TCMs, decreased by 14.33% as compared with those in 2018; 1,005 registration applications were for biological products, increased by 23.3% as compared with those in 2018. The details of acceptance of registration applications for chemical drugs, TCMs and biological products requiring technical review in 2016- 2019 are shown in Figure 2.
CDE has accepted a total of 700 applications (involving 319 varieties, increased by 20.8% as compared with those in 2018) for the registration of Class 1 innovative drugs (the number of varieties of chemical drugs is based on the statistical analysis of active ingredients, while the varieties of TCMs and biological products are all counted by their generic names, the same below), covering 302 INDs (increased by 26.4% as compared with those in 2018) and 17 NDAs (decreased by 8 varieties as compared with those in 2018) for Class 1 innovative drugs.
Review and approval for drug registration applications
1. Completion of review and approval annually
From 2015 to 2018, CDE basically solved the backlog of drug registration applications by reinforcing review power and improving the efficiency of review from all aspects such as expanded review channels, enhanced review project management, large-scale recruitment, seconded personnel from provincial bureaus, and other measures. The work focus of CDE gradually shifted from reducing the backlog of registration applications to improving the rate of on-time review and approval of drug registration applications. In 2019, CDE accomplished over 90% of on-time review & approval of registration applications for TCMs, chemical drugs and biological products, thus basically completed the work objective set forth in the Opinions of the State Council on Reforming the Review & Approval System for Drugs and Medical Devices (State Council [2015] No.44, hereinafter referred to as Document No.44).
In 2019, a total of 8,730 registration applications (including 5 drug-device combination products) were subject to review and approval, of which 6817 applications were subject to technical review (including 4,075 applications subject to technical review and administrative approval of CDE), and the rest 1,908 applications were subject to direct administrative approval. The number of registration applications subject to review and approval and those pending for review and approval has dropped from nearly 22,000 at the peak of September 2015 to 4,423 (excluding those applications whose review have been completed and are pending for the Applicants' supplementary materials) by the end of 2019, consolidating the reform accomplishment of liquidating the backlog of registration applications as required by Document No.44.
In 2019, among the 4,423 registration applications subject to review and approval and those pending for review and approval, 3,334 applications were initiated for review, 450 applications were pending for verification at the end of the review; and the remaining 639 review tasks covered 290 applications subject to suspended review with time-keeping while waiting for related varieties, and 235 applications waiting for the applicant to verify the quality standards, package inserts and labels, and 36 applications waiting for test reports.
Among the 6,817 registration applications with completed technical review, 300 applications were for TCMs , 1104 applications were for biological products, and 5,413 applications were for chemical drugs (accounting for about 79% of all completed reviews).
2. Completion of reviews for various types of registration applications
CDE completed the reviews for 1,001 IND applications (including 1 drug-device combination product), 270 NDA reviews (including 1 drug-device combination product), and 1,664 ANDA reviews (including 3 drug-device combination products).
3. Approved reviews
In 2019, CDE reviewed and approved 926 IND applications, 164 NDAs, 654 ANDAs, and 260 applications for consistency evaluation of oral solid preparations (95 varieties based on the statistical analysis of active ingredients, and 107 varieties counted by their generic names), and the number of varieties was increased by 66.7% compared with that in 2018 (57 varieties).
CDE reviewed and approved the marketing of 10 varieties of Class 1 innovative drugs, and 58 varieties of imported brand-name drugs (including new indications).