Annual Report for National Medical Device Adverse Event Monitoring (2019) Released
To fully reflect the monitoring of adverse events in relation to China's medical devices in 2019, the National Center for Adverse Drug Reaction Monitoring has compiled and released on April 26, 2020 the Annual Report for National Medical Device Adverse Event Monitoring (2019).
Progress of medical device adverse event monitoring
In 2019 , China' s medical device adverse event monitoring continued to adhere to the Four Strictest (Strictest Standards, Regulation, Punishment, and Accountability) requirements, and implemented the Provisions for Monitoring and Re-evaluating the Adverse Events of Medical Devices (hereinafter referred to as the Provisions). Taking the evaluation of medical device risks as the main line, and focusing on implementing the principal responsibility of adverse event monitoring by medical device registrants and applicants for record filing (hereinafter collectively referred to as registrants), we furthered the system construction, continuously developed the approaches for relevant publicity and training, explored in depth the monitoring and evaluation methods, and comprehensively improved risk early warning and disposal capabilities. New progresses have been made in the monitoring of medical device adverse events:
(I) Collection of medical device adverse event reports
In 2019, the National Medical Device Adverse Event Monitoring Information System received more than 390,000 reports of suspected medical device adverse events, with an average number of 297 reports per million population; 96.70 percent of districts and counties across China reported medical device adverse events, and the registered users at the grassroots level of the system topped 310,000, covering 19,662 medical device registrants. Compared with 2018, the number of medical device adverse event reports nationwide has remained stable, the number of registered users at the grassroots level of the system has continued to increase, and a smooth transition has been achieved from the old to the new medical device adverse event monitoring information system.
(II) Risk disposal of medical device adverse events
In 2019, the risk signal evaluation & disposal of medical device adverse events has been carried out in depth. We've strengthened the daily monitoring, early warning analysis and quarterly summary of national medical device adverse event reports. Based on spotted risk conditions, 3 issues of Medical Device Adverse Event Information Notification and 12 volumes of Medical Device Pharmacovigilance Expresses were released in 2019. The intensive monitoring of medical device adverse events continued to advance in the 13th Five-Year Plan period, and each undertaking unit actively reviewed the previous work, sorted out product risks, and ensured the orderliness of key monitoring.
(III) Improvement of medical device monitoring capabilities
In 2019, the National Center for ADR Monitoring trained a total of more than 1,300 person-time for registrants, medical institutions, and monitoring agencies. At the same time, the Center provided teachers for Workshops organized by drug regulatory authorities at all levels on the Provisions and relevant guidance to underscore the principal responsibility of registrants and improve the capacity level of monitoring personnel, and the training results were substantial. Furthermore, the Center actively followed up IMDRF's (International Medical Device Regulatory Agency Forum) work progress on adverse event terminology & coding and patient registration data evaluation. It officially joined the national regulatory authority reporting exchange mechanism to further go global.
General situation of national medical device adverse event reporting
(I) Overview of reporting in 2019
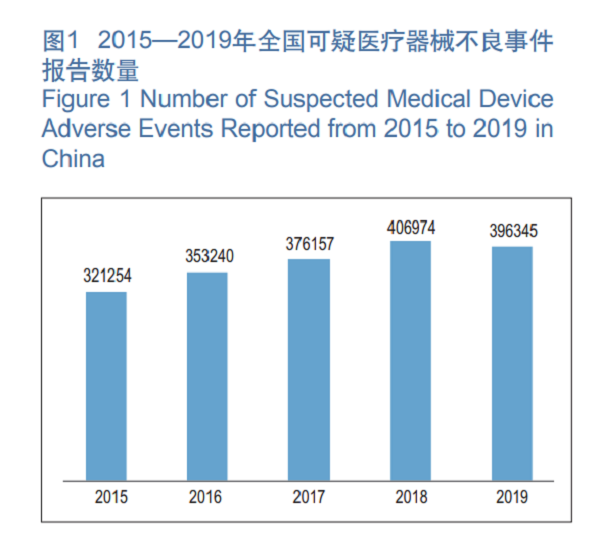
1. Number of Medical Device Adverse Event Report. In 2019, the National Medical Device Adverse Event Monitoring Information System has received a total of 396,345 reports of suspected medical device adverse events, a decrease of 2.61 percent YOY (See Figure 1) .
2. Average number of reports per million population
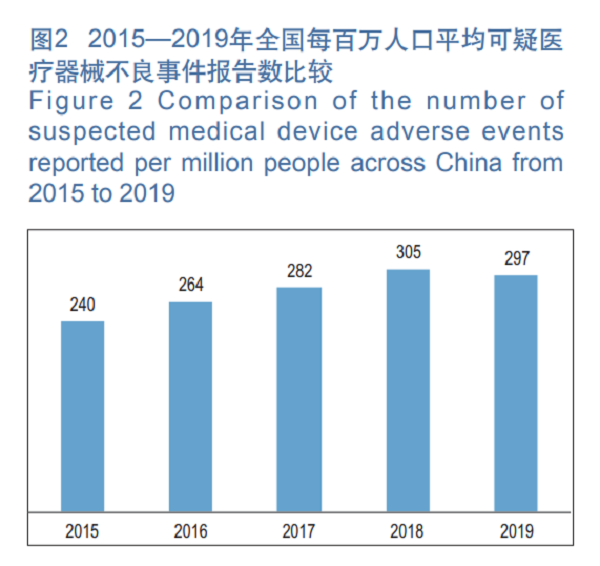
In 2019, the average number of suspicious medical device adverse event reports per million population in China was 297, a decrease of 2.62 percent over the previous year (Figure 2).
3. County-level coverage In 2019, the county-level coverage rate
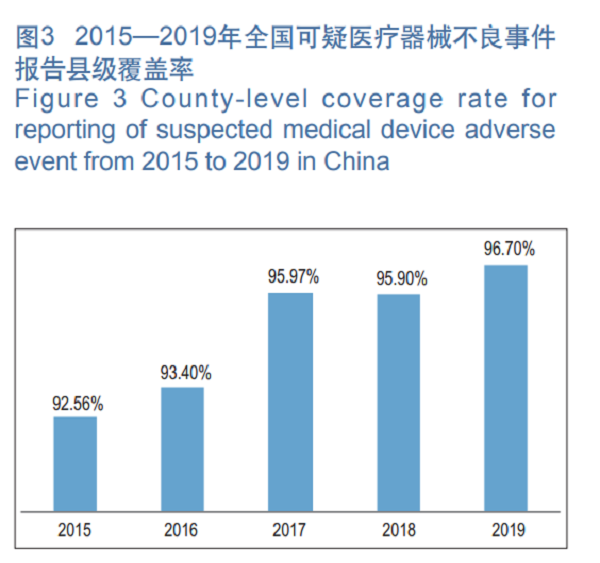
In 2019, the county-level coverage rate for reporting of suspected medical device adverse events in China was 96.70 percent, up by 0.80 percentage points YOY (Figure 3).
(II) Number of registered grassroots users nationwide
As of Dec 31, 2019, there were a total of 318,986 grassroots user units (incl. registrants, distributors and user units) registered in the National Medical Device Adverse Event Monitoring Information System, covering 19,662 registrants (6.16%); 178,295 distributors (55.89%); and 121,029 user units (37.94%).
In 2019, the total number of registered grassroots users increased by 15.69 percent over the previous year. Among them, the registered grassroots users of registrants, distributors and user units have increased by 41.92 percent, 24.22 percent and 2.28 percent over 2017, respectively.



