The 2018 Drug Review Annual Report released
On July 1, 2019, the 2018 Drug Review Annual Report was released, which is excerpted as follows:
In 2018, under the strong leadership of the National Medical Products Administration (hereinafter referred to as NMPA), the Center for Drug Evaluation (hereinafter referred to as CDE) has, pursuant to the Opinions of the General Office of the CPC Central Committee and the General Office of the State Council on Deepening the Reform of Examination & Approval System to Encourage Innovation in Drugs and Medical Devices (General Office [2017] No. 42, hereinafter referred to as Document No. 42); the State Council's Opinions on Reforming the Review & Approval System for Drugs and Medical Devices (State Council [2015] No. 44 (hereinafter referred to as Document No. 44), and the requirements of the Standing Committee of the State Council on April 12 and June 20, performed a series of tasks to encourage innovation in drug R&D, improve drug quality, and guarantee drug safety, effectiveness and accessibility, etc. CDE has furthered the reform of the drug review & approval system with high responsibility and sense of mission, adhered to the law-based, scientific and standardized review, to resolutely safeguard and promote public health.
I.Acceptance of drug registration applications
In 2018, CDE accepted a total of 7,336 new registration applications (counted by acceptance numbers, the same below), of which 5,574 are subject to technical review, the rest to direct administrative examination and approval (dispense with technical review, the same below). Compared with 2017, the number of registration applications requiring technical review by CDE saw a substantial increase (up by 47%) in 2018, whereof the numbers of applications for registration of TCMs, chemicals and biologicals all saw a significant increase (up by 30%, 50% and 42%, respectively).
In 2018, CDE accepted the registration applications for a total of: ---264 varieties of innovative drugs (involving 533 acceptance numbers, the number of varieties of chemical drugs is based on the statistical analysis of active ingredients, while the varieties of TCM and biological products are all counted by their generic names), up by 21% YOY, covering 239 INDs (up by 15% YOY) and 25 NDAs (up by 150% YOY) for Class 1 innovative drugs.
--- 157 varieties of Class 1 chemicals, covering 16 NDAs (up by 100% YOY) for Class 1 innovative chemical drugs.
--- 37 varieties of Class 1-6 new TCMs, covering 8 NDAs (increased 8 times than that in 2017); and 29 INDs, one of which went for Class 1 TCM innovative drugs.
--- 106 Class 1 innovative biologicals (an increase of 62% over 2017, involving a total of 123 acceptance numbers for 6 preventive biologicals and 117 therapeutic biologicals), covering 9 NDAs (involving 11 acceptance numbers, 2 for preventive biologicals and 9 for therapeutic biologicals), increased 5.5 times than that in 2017.
(I)Overall situation
Of the 7,336 new registration applications accepted by CDE, those for chemical drugs accounted for 82% (5,979) of the total. For a four-year comparison, see Figure 1 for details.
Among the 5,574 applications requiring technical review, 4,459 were for chemical drugs, accounting for 80% of all those for technical reviews, and 300 and 815 were for TCMs and biologicals, respectively.
(II)Acceptance of domestically produced innovative drugs
CDE accepted 448 applications for the registration of domestically-produced Class 1 innovative drugs (involving 222 varieties), of which 403 were for INDs (involving 198 varieties), and 45 were for NDAs (involving 24 varieties). As per drug-specific statistics, 323 applications were for chemical drugs (involving 115 varieties), 2 for TCMs(involving 1 variety), and 123 biologicals (involving 106 varieties). The indications of innovative drugs are mainly concentrated in the field of anti-tumor and endocrine systems, and digestive systems.
(III)Imported drugs
CDE accepted 75 applications for registration of Class 5.1 imported chemical brand- name drugs (involving 50 varieties), and 85 applications for registration of imported innovative drugs (incl. 42 varieties). The indications for innovative drugs are mainly focused on anti-tumor, circulatory and digestive systems.
(VI) Acceptance of various types of registration applications
1.Chemical drugs
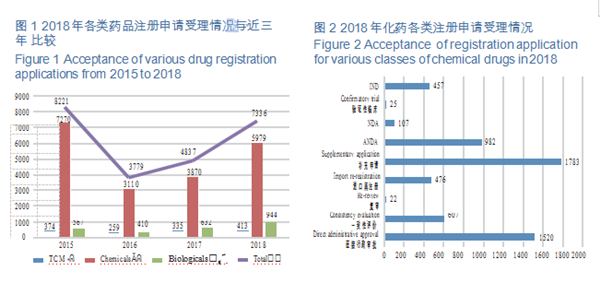
CDE accepted 5,979 applications for registration of chemical drugs, of which 107 were for NDAs, an increase of 43% compared with 2017; and 982 applications were for ANDAs (generic drugs), an increase of 79% compared with 2017. The details of the acceptance of registration application for various classes of chemical drugs are shown in Figure 2. The 2015-2018 acceptance of registration applications for clinical trial, marketing and consistency evaluation of chemical drugs is detailed in Figure 3.
(1)Innovative chemicals
CDE accepted registration applications for 157 varieties of Class 1 innovative chemicals, covering 16 innovative NDAs (up by 100% YOY), 115 varieties of domestically produced innovative chemicals, and 42 imported ones. For such details from 2015 to 2018, see Figure 4.
(2)Indications for chemical INDs
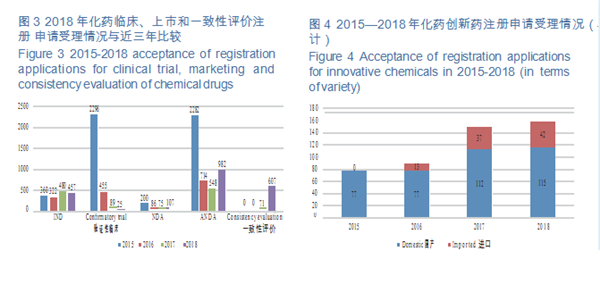
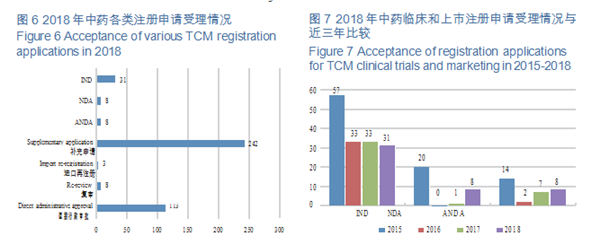
CDE accepted 457 applications for chemical INDs, of which 325 were for domestic ones, and 132 for imported ones. The indications for domestic IND applications are mainly concentrated in the fields of anti-tumor, endocrine and digestive systems; those for imported IND applications are mainly concentrated in the fields of anti-tumor, endocrine system and circulatory system. The specific therapeutic areas are shown in Figure 5.
2.Acceptance of TCM registration applications
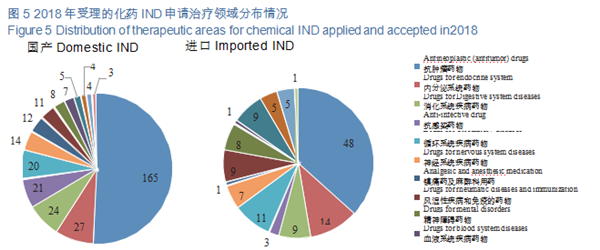
CDE accepted 413 TCM registration applications, covering 31 applications for
INDs, 8 NDAs, and 8 ANDAs. The details of the acceptance of registration applications for various TCMs are shown in Figure 6. The 2015-2018 acceptance of registration applications for TCM clinical trials and marketing is detailed in Figure 7.
(1)New TCMs
CDE accepted 39 applications for registration of Class 1-6 new TCMs, covering 8 NDAs (8 varieties involved), increasing 8 times than that in 2017; 31 INDs (incl. 29 varieties), 2 of which were for Class 1 innovative TCM (involving 1 variety).
(2)Indications of TCM INDs
CDE accepted 31 applications for TCM INDs, 65% of which are with therapeutic areas mainly covering digestion, cardiovascular, respiratory and psychoneural system.
3.Biological products
CDE accepted 944 applications for registration of biological products, covering 298 INDs; and 85 NDAs, up by 70% YOY. For such details, see Figure 8. The acceptance of registration applications for clinical trials and marketing of biologicals in 2015-2018 is detailed in Figure 9.
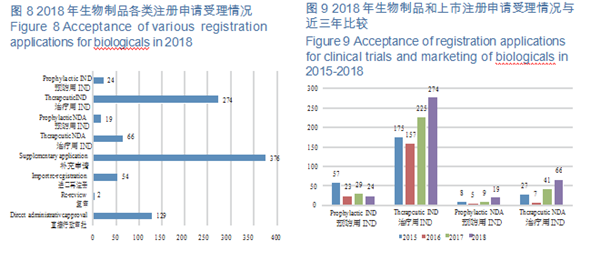
(1)Class 1 innovative biologicals CDE accepted 123 applications for registration of Class 1 innovative biologicals (incl. 6 prophylactic biologicals and 117 therapeutic ones), an increase of 62% over 2017, covering 11 Class 1 NDAs (incl. 2 prophylactic products and 9 for therapy, involving 9 varieties), increasing 5.5 times than that of 2017; 112 Class 1 INDs (incl. 4 prophylactic products and 108 therapeutic ones, involving 97 varieties), an increase of 51% over 2017.
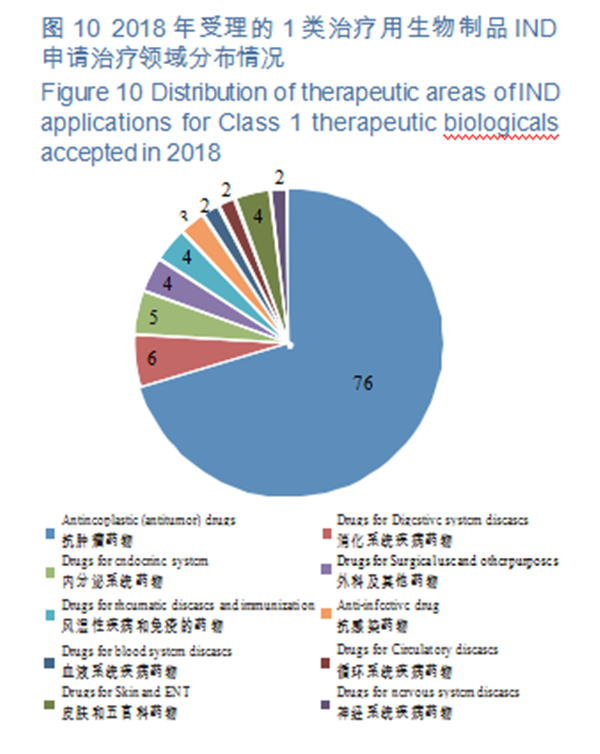
(2)Indications of INDs of Class 1 innovative biologicals for therapeutic use CDE accepted 108 IND applications for Class 1 therapeutic biological products (involving 93 varieties), 70% of which have indications mainly concentrated in the field of anti-tumor treatment, for specific therapeutic areas, see Figure 10.
II.Review & approval of drug registration applications
(I)Overview of accomplished review & approval
1.Review & approvals accomplished in 2018
As of the end of 2018, CDE had accomplished over 90% of the review & approval of registration applications for TCM, chemicals and biologicals, thus basically completed the objective set forth in Doc. No. 44 requiring the completion of review & approval in 2018 within the prescribed time limit. Of the grand sum of 9,796 registration applications reviewed and approved in 2018, 7,988 were subject to technical review (incl. 4,052 administrative approval tasks requiring technical review), and 1,808 to direct administrative approval.
The number of pending registration applications has dropped from nearly 22,000 at the peak of September 2015 to 3,440 (excluding those applications whose review have been completed and are pending for the Applicants' supplementary dossiers) by the end of 2018, further consolidating the accomplishment of liquidating the backlog of registration applications as required by Doc. No. 44. The changes in the number of registration applications queued up for review & approval in 2014-2018 are shown in Figure 11.
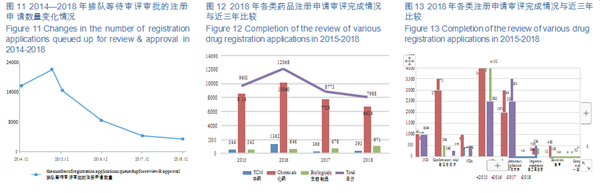
Of the applications with completed review, 6,624 were registered for chemical drugs, accounting for about 83%. The completion status of various types of drug registration applications in 2015-2018 is detailed in Figure 12.
2.Completed category-specific reviews for registration application
In 2018, CDE completed the reviews for 1,094 IND applications, 296 NDAs, and 2,388 ANDAs. The completion of the review of various drug registration applications in 2015-2018 is detailed in Figure 13.
3.Approved reviews
In 2018, CDE reviewed and approved (in the Annual Review Report of previous years, it was stated as passed the review and recommended for approval, the same below) 947 INDs, 175 NDAs, and 1,038 ANDAs.
CDE reviewed and approved the marketing of 9 varieties of Class 1 innovative drugs, and 67 varieties of imported brand-name drugs. For details, see Annexes 1 and 2.
(II)Completion of the review of chemicals registration applications
1.Overall situation
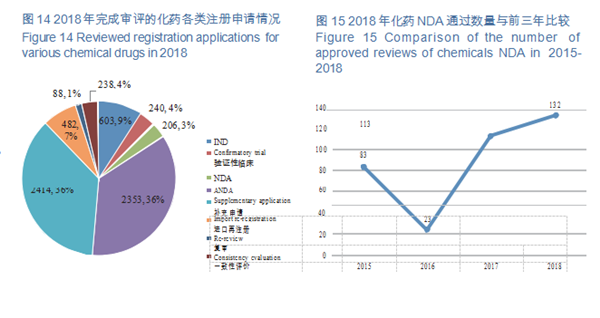
CDE completed the review of registration applications for 6,624 chemical drugs, including 843 clinical applications (IND and confirmatory trial), 206 NDAs, and 2,353 ANDAs. See Figure 14 for details of the various registration applications for chemical drugs that have been reviewed.
2.Approved reviews
CDE completed 206 reviews of chemicals NDA, 132 (in terms of acceptance number) of which were approved, a comparison with the previous three years is shown in Figure 15. For details of various registration applications for chemical drugs with completed reviews in 2018, see Table 1.
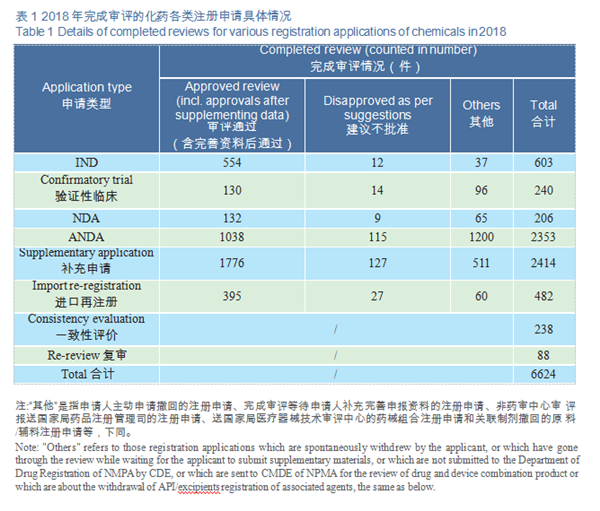
CDE completed reviews for 603 applications for chemicals IND, 554 of which were approved, including 449 IND applications for Class 1 innovative drugs (involving 172 varieties). The numbers of IND approved for Class 1 chemical innovative drugs in 2015-2018 (in terms of variety) are shown in Figure 16.
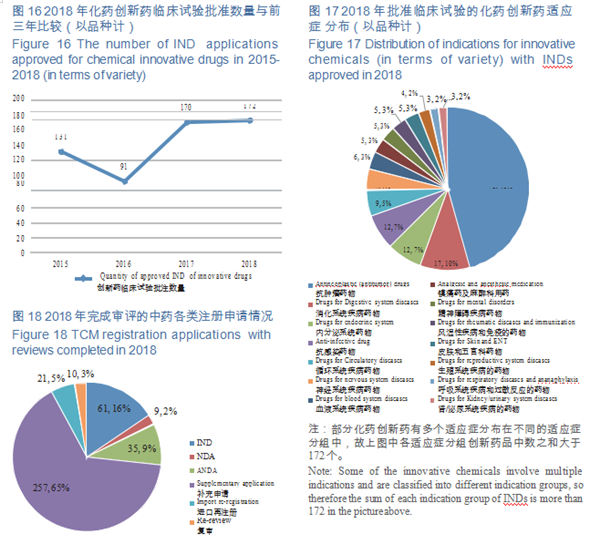
CDE reviewed and approved the INDs of 172 varieties of innovative drugs, mostly for anti- tumor, digestive system, endocrine system and anti-infective use, accounting for 68% of the total. The distribution of indications for innovative chemicals (in terms of variety) with approved INDs is shown in Figure 17.
(III)Completion of the review of TCM registration application
1.Overall situation
CDE completed 393 reviews of TCM registration applications, covering 61 INDs, 9 NDAs, and 35 ANDAs. See Figure 18 for details of the various TCMs registration
applications with completed reviews.
2.Approved reviews
CDE reviewed and approved 44 TCM IND applications; 2 TCM NDAs (involving 2 varieties: Guanhuangmu Particles, Jinrong Particles). The details of completed reviews for various TCM registration applications are shown in Table 2. The comparison of TCM IND approval and NDA approval (in terms of acceptance number) from 2015 to 2018 is shown in Figure 19.
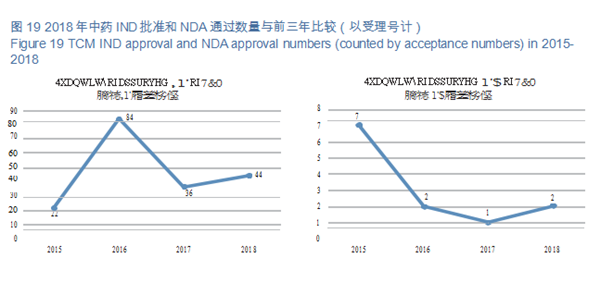
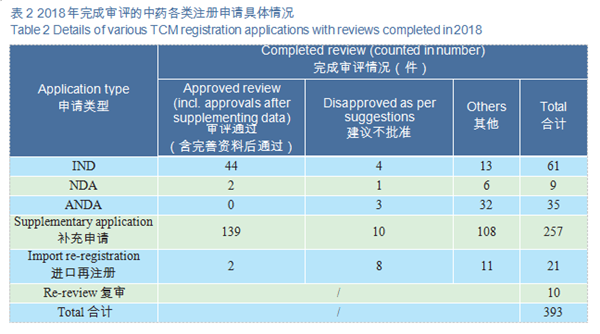
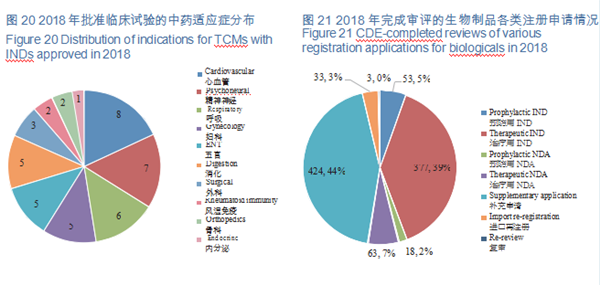
CDE reviewed and approved 44 TCM IND applications, involving 10 indications covering cardiovascular, psychoneural, and respiratory systems, accounting for 48%. The specific therapeutic areas are shown in Figure 20.
(IV)Completion of the review of registration application for biologicals
1.Overall situation
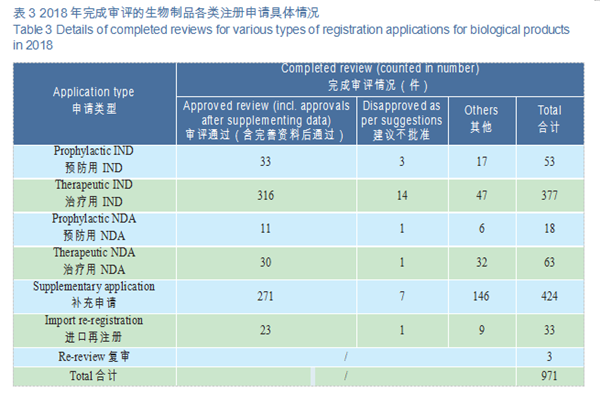
A total of 971 applications for biologics registration were completed by CDE, covering 53 IND applications for prophylactic biological products (prophylactic IND), 377 IND applications for therapeutic biological products (therapeutic IND), 18 NDAs for prophylactic biological products (prophylactic NDA), and 63 NDAs for therapeutic biological products (therapeutic NDA).
See Figure 21 for details of the various registration applications for biologicals that have been reviewed.
2.Approved reviews
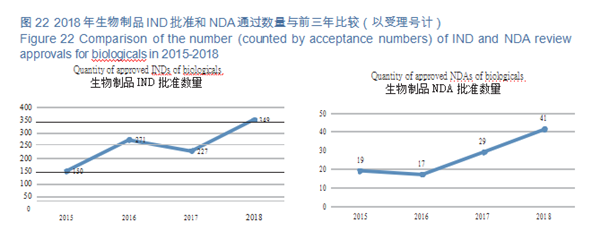
CDE reviewed and approved 33 prophylactic INDs, 316 therapeutic INDs; 11 prophylactic NDAs and 30 therapeutic NDAs. The details of the various registration applications for biological products completed in 2018 are shown in Table 3. The IND and NDA approval of biological products are compared with the previous three years (in terms of acceptance number) as shown in Figure 22.
CDE reviewed and approved 349 biological INDs, for details of the distribution of therapeutic areas of approved therapeutic biological INDs, see Figure 23 (omitted).
(V)Completion of administrative examination and approval tasks
In 2018, CDE completed a total of 5,860 administrative examination and approval tasks, covering 1,808 direct administrative examination and approval tasks (i.e.,supplementary applications dispense with technical review) with an average time limit for examination and approval of 12.3 working days, far less than the statutory 20- day time limit for administrative examination and approval, 1,656 of which were completed within the statutory 20-day time limit, and the average completion rate within time limit was 92% in 2018. CDE completed the administrative examination and approval for 4,052 tasks with technical review (viz., IND, import re-registration and supplementary applications requiring technical review, etc.), with an average time limit of 18.6 working days, which also curtails the statutory 20- day time limit, the average completion rate within time limit was 84% in 2018. (Note:The above-mentioned 4,052 tasks exclude those that have been accepted and reviewed prior to the joint review & approval of APIs, excipients and packaging materials and transferred to registration platform management of APIs, excipients and packaging materials.
(VI)Incorporation of prioritized review.
1.Incorporation of varieties for prioritized review
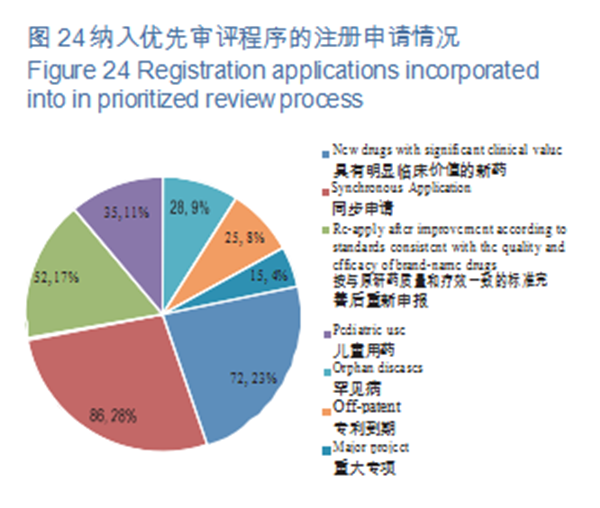
As per the Opinions on Fueling Pharmaceutical Innovation via Prioritized Review & Approval (SYJYHG [2017] No.126) of the former China Food and Drug Administration (hereinafter referred to as the former CFDA), in 2018, CDE will have incorporated a total of 313 registration applications into prioritized review process, covering 63 applications for pediatric use and orphan diseases. Of the registration applications that were included in the prioritized review in 2018, the proportion of Synchronous Applications (which refers to 5th case in the Scope of Prioritized Review & Approval (A) (CFDA No. 19), viz., drugs whose clinical trials have been simultaneously applied and approved in the European Union and the United States, or drugs that are manufactured in China with the same production line, and simultaneously applied for marketing in EU or US and have passed the on-site inspection of their drug evaluation and approval authorities) was the largest, accounting for 28%, followed by new drugs with significant clinical value, accounting for 23%. The registration applications incorporated into prioritized review process are detailed in Figure 24.
2.Completed review of varieties subject to prioritized process
In 2018, a total of 83 varieties (in generic terms) were expedited for marketing approval via prioritized review process, such as Albuvirtide for Injection, Danoprevir Sodium Oral Tablets for hepatitis C treatment, and small-molecule angiogenesis inhibitor Fruquintinib Capsules for the treatment of advanced colorectal carcinoma, and other innovative drugs independently researched and developed in China, the list of specific varieties is shown in Annex 3.
(VII)) Communication and exchanges (omitted)
III.Encouraging innovation and safeguarding medication safety for the public (omitted)
IV.Programs and progresses (omitted)
V.Key objectives for 2019 (omitted)



