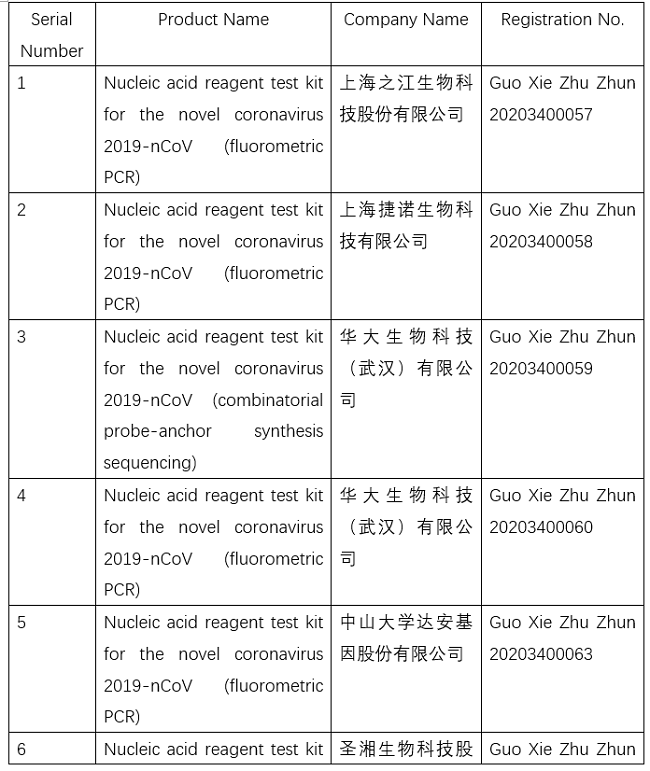
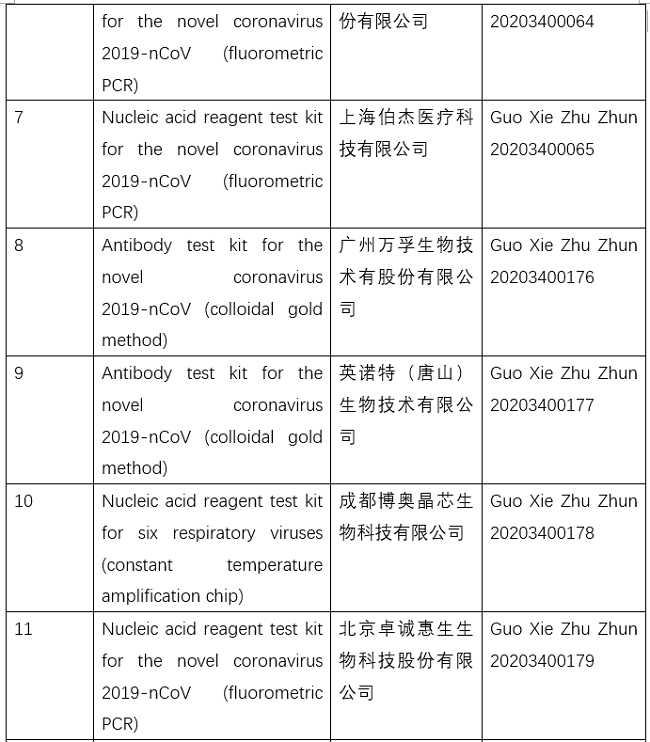
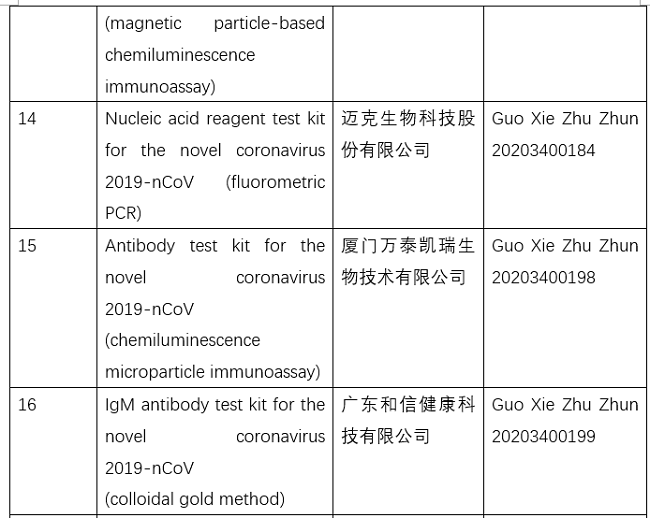
NMPA conducts emergency approval of testing products for 2019-nCoV
The National Medical Products Administration (NMPA) recently approved two IgM/IgG antibody test kits (colloidal gold method).
So far, the NMPA has approved 11 nucleic acid reagent test kits and eight antibody reagent test kits.