NMPA issued the Announcement on the Annual Report for National ADR Monitoring (2018)
On October 18, 2019, NMPA issued the Announcement on the Annual Report for National ADR Monitoring (2018), which is excerpted as follows:
I. Overall Situation of Adverse Drug Reaction Monitoring
From 1998 to 2018, China's national ADR Monitoring has gone through the vicissitudes of two decades, during which NMPA focused on building an ADR monitoring system, improving relevant laws and regulations, expanding monitoring coverage, and establishing an early warning mechanism based on risk prevention and control. ADR monitoring has thus developed rapidly. In 2018, in accordance with the Four Strictest (Strictest Standards, Regulation, Punishment, and Accountability ) requirements put forward by General Secretary Xi Jinping on food and drug safety, adhering to the motif of safeguarding the safety of the people's medication, new progresses have been made in ADR monitoring:
First, reinforce intelligent supervision and further expand the coverage of monitoring. We've ameliorated the national ADR monitoring network system, developed and constructed the monitoring system wherein the Marketing Authorization Holders(MAHs) shall directly report the ADR, and expanded the monitoring coverage. In 2018, 97.9% of the districts and counties reported ADRs, and the national average number of reports per million population reached 1,119, which has achieved the corresponding goal in the 13th Five-Year Plan. We continued to expand our monitoring techniques & approaches and cooperated with medical institutions to build more than 150 monitoring sentinels, providing a solid foundation for monitoring.
Second, conduct in-depth safety evaluation and timely address risk warning signals. Based on monitoring data analysis and evaluation results, in 2018, we've released a total of 33 announcements on the revision of drug package inserts; mandated the cessation of production, sales and use of Pyrithioxine Injections, Composite Terfenadine Tablets, Sulfisomidiae Tablets and Terfenadine, Ibuprofen and Pseudoephedrine Capsules, and issued 12 volumes of Pharmacovigilance Express. We continued to optimize the early warning system and timely handled more than 150 highly concerned ADR-events clustering signals, focusing on early detection, early response, early investigation and early disposal to ensure safety of public drug use.
Third, reinforce the principal responsibility of Marketing Authorization Holders for drug safety. In September 2018, NMPA issued the Announcement on the Direct Reporting of Adverse Reactions by Marketing Authorization Holders (Announcement No. 66 of 2018) and the Announcement on the Issuance of the Guidelines for the Collection and Reporting of Adverse Drug Reactions in Individual Cases (No. 131 of 2018), further reinforced the responsibility of MAHs for drug safety, and put forward specific requirements for monitoring, reporting, analysis and evaluation of the MAHs.
II. ADR/ADE (adverse drug event) reporting
(A) Overview of reporting
1. 2018 Annual ADR/ADE Reporting
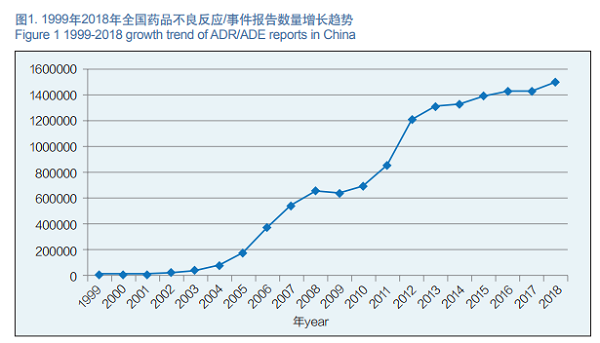
In 2018, the National ADR Monitoring Network received 1.499 million copies of the Report on Adverse Drug Reactions/Events. From 1999 to 2018, the National ADR Monitoring Network received a total of 13.68 million copies of the ADRs / ADEs Report Forms (Figure 1).
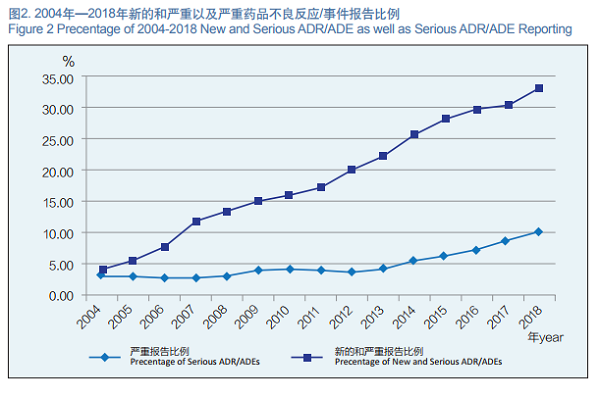
2. New and Serious ADR/ADE Reporting
In 2018, the National ADR Monitoring Network received 495,000 new and serious ADR/ADE reports, accounting for 33.1% of the total number reported in the same period. The steady growth of the proportion of new and serious ADR/ADE reports indicates a constant upclimbing of the availability of ADR reports in China.
In 2018, the National ADR Monitoring Network received 149,000 reports of serious ADRs/ADEs, accounting for 10.0% of the total number of reports in the same period (Figure 2).