The 2018 Drug Review Annual Report released
(III) Completion of the review of TCM registration application
1. Overall situation
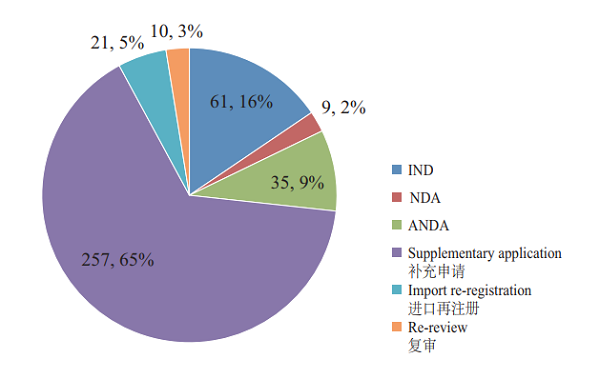
CDE completed 393 reviews of TCM registration applications, covering 61 INDs, 9 NDAs, and 35 ANDAs. See Figure 18 for details of the various TCMs registration applications with completed reviews.
Figure 18 TCM registration applications with reviews completed in 2018
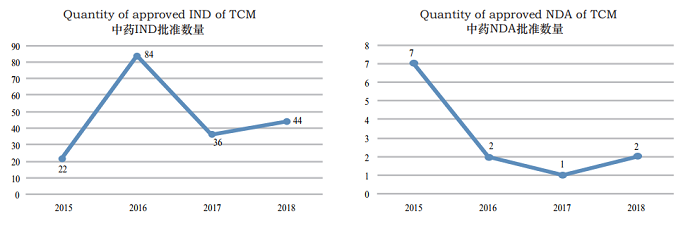
2. Approved reviews CDE reviewed and approved 44 TCM IND applications; 2 TCM NDAs (involving 2 varieties: Guanhuangmu Particles, Jinrong Particles). The details of completed reviews for various TCM registration applications are shown in Table 2. The comparison of TCM IND approval and NDA approval (in terms of acceptance number) from 2015 to 2018 is shown in Figure 19.
Table 2 Details of various TCM registration applications with reviews completed in 2018
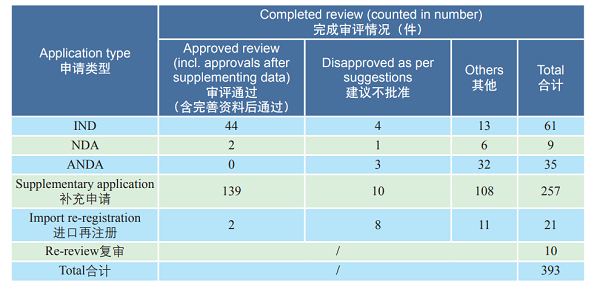
Figure 19 TCM IND approval and NDA approval numbers (counted by acceptance numbers) in 2015- 2018
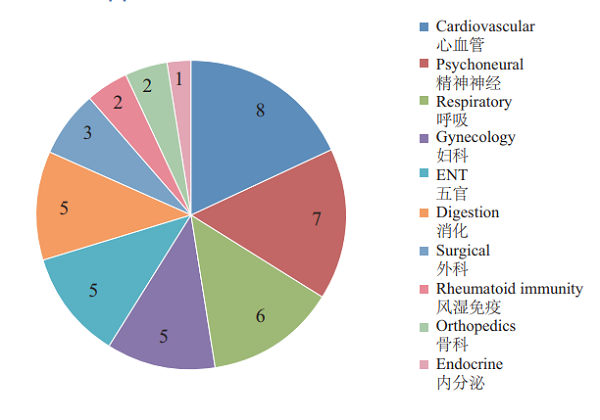
CDE reviewed and approved 44 TCM IND applications, involving 10 indications covering cardiovascular, psychoneural, and respiratory systems, accounting for 48%. The specific therapeutic areas are shown in Figure 20.
Figure 20 Distribution of indications for TCMs with INDs approved in 2018