The 2018 Drug Review Annual Report released
(II) Acceptance of domestically produced innovative drugs
CDE accepted 448 applications for the registration of domestically-produced Class 1 innovative drugs (involving 222 varieties), of which 403 were for INDs (involving 198 varieties), and 45 were for NDAs (involving 24 varieties). As per drug-specific statistics, 323 applications were for chemical drugs (involving 115 varieties), 2 for TCMs (involving 1 variety), and 123 biologicals (involving 106 varieties). The indications of innovative drugs are mainly concentrated in the field of anti-tumor and endocrine systems, and digestive systems.
(III) Imported drugs
CDE accepted 75 applications for registration of Class 5.1 imported chemical brandname drugs (involving 50 varieties), and 85 applications for registration of imported innovative drugs (incl. 42 varieties). The indications for innovative drugs are mainly focused on anti-tumor, circulatory and digestive systems.
(VI) Acceptance of various types of registration applications
1. Chemical drugs
CDE accepted 5,979 applications for registration of chemical drugs, of which 107 were for NDAs, an increase of 43% compared with 2017; and 982 applications were for ANDAs (generic drugs), an increase of 79% compared with 2017. The details of the acceptance of registration application for various classes of chemical drugs are shown in Figure 2. The 2015-2018 acceptance of registration applications for clinical trial, marketing and consistency evaluation of chemical drugs is detailed in Figure 3.
Figure 2 Acceptance of registration application for various classes of chemical drugs in 2018
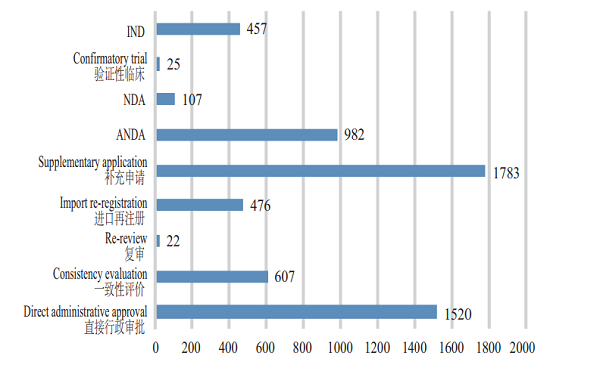
Figure 3 2015-2018 acceptance of registration applications for clinical trial, marketing and consistency evaluation of chemical drugs
(1) Innovative chemicals
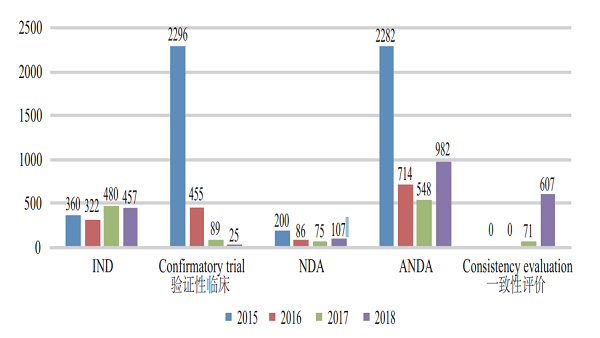
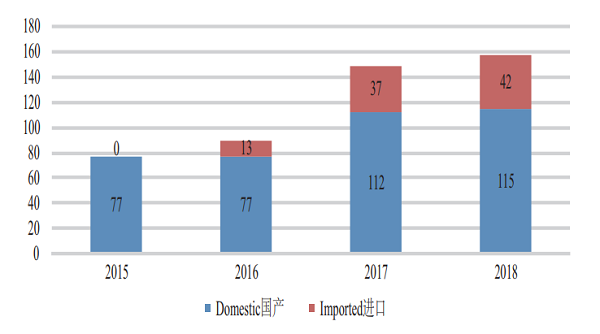
CDE accepted registration applications for 157 varieties of Class 1 innovative chemicals, covering 16 innovative NDAs (up by 100% YOY), 115 varieties of domestically produced innovative chemicals, and 42 imported ones. For such details from 2015 to 2018, see Figure 4.
Figure 4 Acceptance of registration applications for innovative chemicals in 2015-2018 (in terms of variety)
(2) Indications for chemical INDs
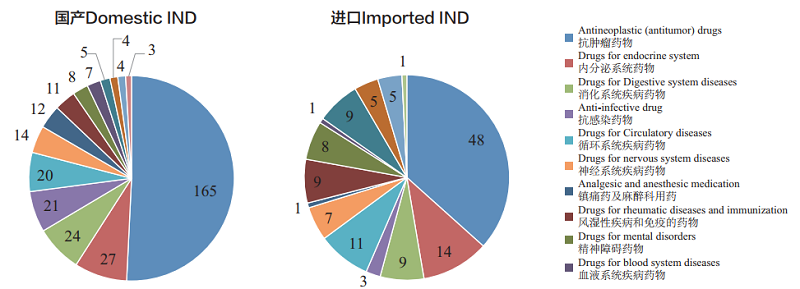
CDE accepted 457 applications for chemical INDs, of which 325 were for domestic ones, and 132 for imported ones. The indications for domestic IND applications are mainly concentrated in the fields of anti-tumor, endocrine and digestive systems; those for imported IND applications are mainly concentrated in the fields of anti-tumor, endocrine system and circulatory system. The specific therapeutic areas are shown in Figure 5.
Figure 5 Distribution of therapeutic areas for chemical IND applied and accepted in 2018