2018 Annual Report for Medical Device Registration Released
On May 31, 2019, NMPA issued the 2018 Annual Report for Medical Device Registration, which consists of five parts, namely, the situation of medical device registration; the acceptance of medical device registration applications; review and approval of medical device registration; review and approval of registration for innovative medical devices and other products; and management of other registration affairs. The statistics period of this Report spans from January 1, 2018 to December 31, 2018.
Figure: Pie-chart of registration forms of medical device registration acceptance
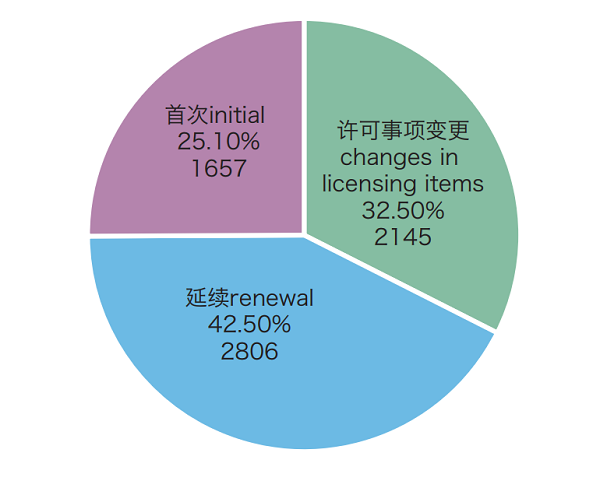
Among them, in 2018, within its purview, NMPA accepted 6608 applications for initial registration, registration renewal and registration of changes in licensing items for medical devices, decreased by 3.3% as compared with that in 2017.
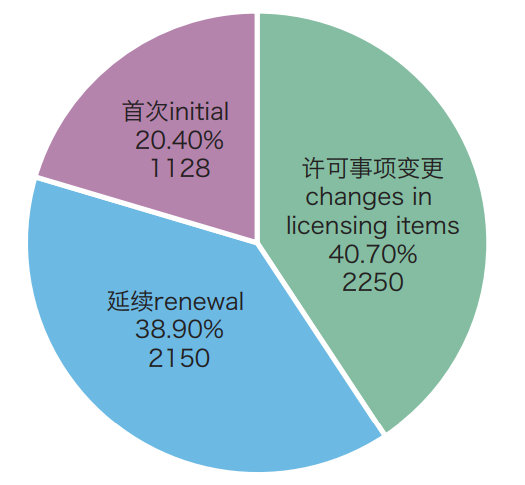
In 2018, NMPA approved a total of 5,528 items of medical device initial registrations, registration renewals and registration of changes. Compared with 2017, the total number of registration approvals is down by 38.0%. Among them, 1,128 were for initial registrations, 2,150 were for registration renewals, and 2,250 were for registration of changes in licensing items.
Figure: Pie-chart of registration forms of approved medical device registrations in 2018
In 2018, NMPA has rejected a total of 118 medical device registration applications, while 238 applications were voluntarily withdrawn by the enterprises.



