NMPA continues emergency approval of testing products for novel coronavirus
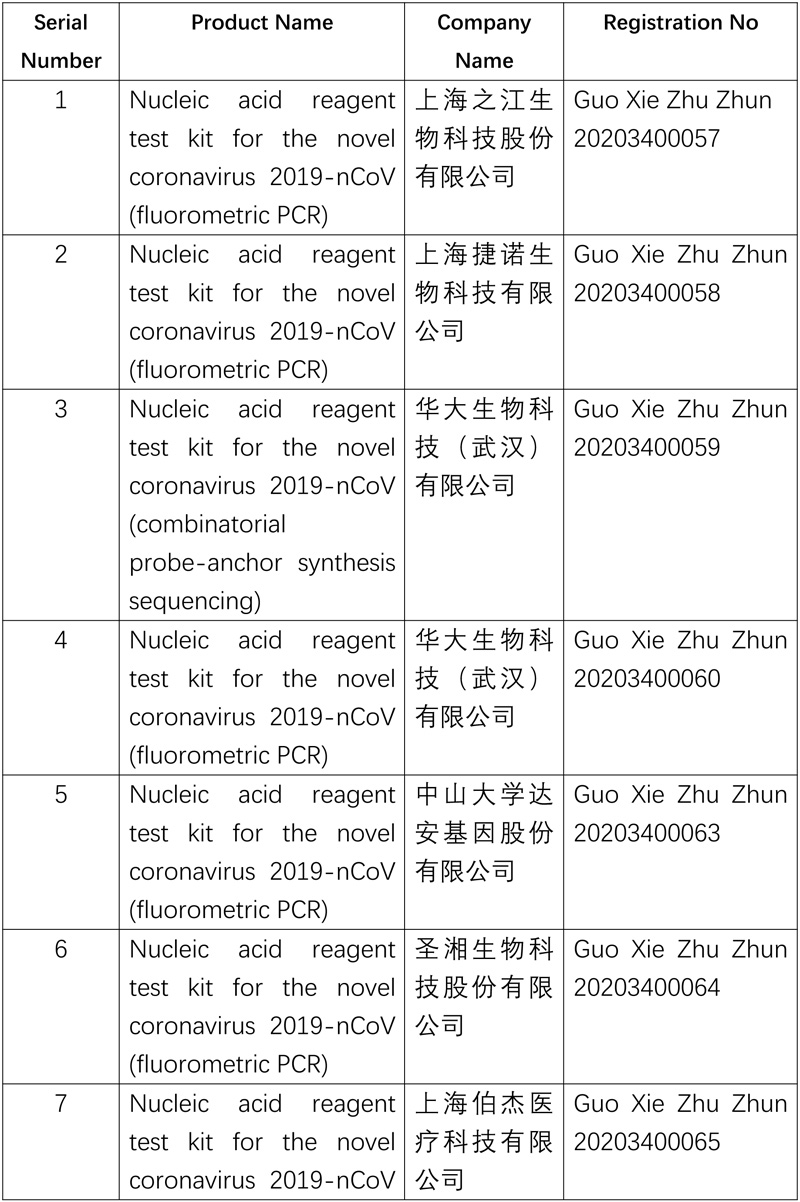
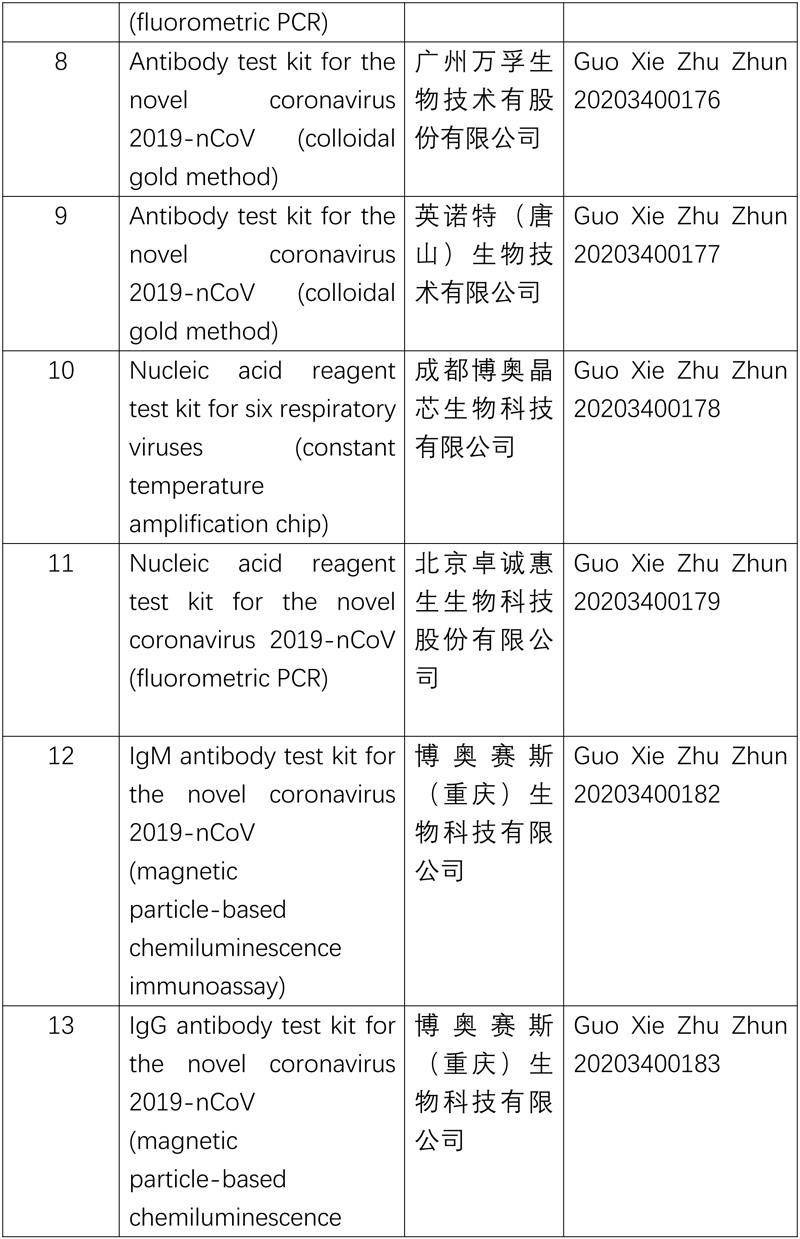
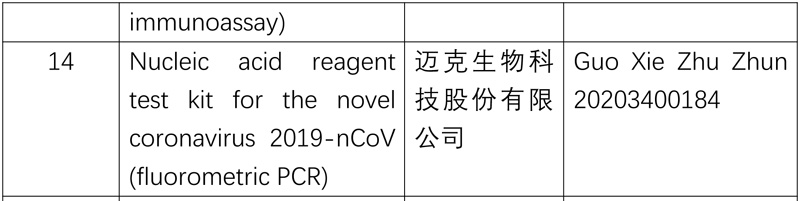
The National Medical Products Administration (NMPA) recently approved four coronavirus testing products developed by three enterprises through its emergency review and approval process, including two antibody reagent (chemiluminescence) test kits and two types of nucleic acid reagent (fluorometric PCR) test kits, further diversifying the testing methods for novel coronavirus, increasing the supply of test kits and enhancing epidemic prevention and control.
So far, ten nucleic acid reagent test kits and four antibody reagent test kitss for the novel coronavirus have been approved.
The NMPA will continue its emergency approval of needed products as the epidemic develops.
The list of approved reagent test kits is as follows: